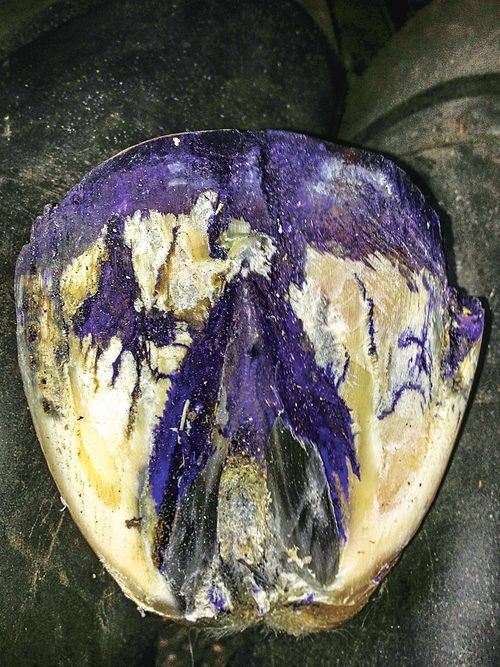
A popular antiseptic dye that’s used in the treatment of equine fungal infections is being pulled from the Canadian marketplace over fears of increased cancer risk.
Health Canada has recalled all products — including equine thrush treatments — that contain gentian violet from the Canadian marketplace. The registered veterinary drug products that contain gentian violet — Blu Kote, Dr. Naylors, Cristisol, Guard and Wound Spray, and Wound Clear Spray — have voluntarily agreed to stop marketing its products. In addition, their product licenses have been canceled and the products have been recalled in Canada.
“Given the seriousness of this risk, Health Canada is advising Canadians to stop using all human and veterinary drug products containing gentian violet,” according to a statement from Health Canada. “There is no safe level of these products, and therefore any exposure to these products is a potential cause for concern.”
You May Also Be Interested In...
How to Identify and Treat Thrush
Thrush is a degenerative condition of the central and lateral sulci of the frog generally caused by a bacterial infection. To learn more about equine thrush, how to prevent it and treat it, read “How to Identify and Treat Thrush.” Read now »
The World Health Organization’s Codex Alimentarius Commission recommended Health Canada review the potential risk of cancer associated with veterinary drug residues in foods. However, the agency took it a step further and reviewed the safety of human non-prescription drugs, veterinary drugs and medical devices containing gentian violet. As a result of two reviews, Health Canada is recommending:
• The immediate halt to using all drug products that contain gentian violet, including on animals. Human products should be returned to a pharmacy for disposal. Animal owners are urged to discuss options for safe disposal with their veterinarians or local pharmacy.
• Do not use dressings containing gentian violet for longer than 6 months or if you are pregnant or nursing.
• Consult with a health professional if you have used any health products that contain gentian violet and have health concerns.
• Read product labels to determine whether products have been authorized for sale by Health Canada. Authorized health products have an eight-digit Drug Identification Number (DIN), Natural Product Number (NPN) or Homeopathic Drug Number (DIN-HM). You can also check whether products have been authorized for sale by searching the Drug Product Database, Medical Devices Active Licence Listing (MDALL) and Licensed Natural Health Product Database.
• Report any health product-related adverse reactions or complaints to Health Canada.
• If you find a product containing gentian violet in the Canadian marketplace, contact Health Canada at (800) 267-9675 or complete an online complaint form.
Although the new findings suggest a risk for cancer, researchers published a scientific study in the Journal of the American Medical Association last fall that concluded gentian violet kills some cancer cells. The in vitro study found that gentian violet attacked tumor viability and growth in cutaneous T-cell lymphoma.








Post a comment
Report Abusive Comment